Simplifying Automated Microfluidics
By Stefan Martin, Mechanical Engineering Team & Mike Fussell, Marketing Team
Published on May 24, 2022
Microfluidic systems hold tremendous promise for high-throughput screening and the development of rapid, highly sensitive multi-omics medical diagnostics. Organ-on-a-chip (OOAC) biomimetic systems are enabling new approaches to translational research and drug discovery. High-throughput analysis of live cells and unparalleled reagent efficiency and process control can reduce reagent consumption and experiment time by orders of magnitude compared to traditional laboratory protocols. This makes automated microfluidics an invaluable tool for both research and commercial applications.
Getting started with automated microfluidics can be a time consuming and complicated task - particularly on a limited budget. Fortunately, Zaber’s portfolio of motion control and microscopy products coupled with freely available Python libraries makes this technology more accessible than ever. To demonstrate this, we built a demonstration system capable of automated sample positioning, fluid delivery and imaging of a microfluidic device (Fig. 1). In this article, we describe the components we selected to build this effective and flexible solution. The microscopy modules are from the Nucleus® automated microscopy platform which provides a complete set of interchangeable hardware modules and software tools for building your bespoke inverted or upright standalone microscope or optical subsystem.
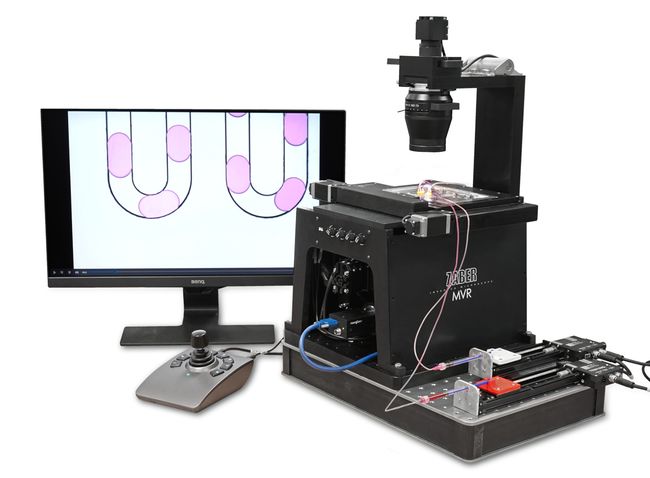
Figure 1. Automated microfluidic system built using a Nucleus MVR inverted microscope for automated chip positioning and imaging, and X-LSM linear stage-driven syringe pumps for automated fluid delivery.
Requirements
To fully realize the benefits of automation, a system should be capable of:
Automated chip alignment. Diagnostic chips are typically single use. Automated positioning of chips improves throughput by eliminating manual alignment of the world-to-chip interface and key on-chip features, particularly when using high magnification optics.
Automating fluid delivery. Precise control over the flow rate and timing of fluid delivery is critical to the correct operation of the microfluidic device by ensuring the optimum flow rates, and delivery sequence of reagents and samples.
Automating image acquisition. Imaging enables validation of the operation of devices, feedback control of flow rates and droplet sizes. Automating image acquisition can deliver significant cost-savings by enabling advanced imaging techniques which eliminate the need for expensive high-speed cameras.
Component Selection
Zaber X-series products are ideal building blocks for developing an automated microfluidic system. Their common control API greatly simplifies the software setup, while their support for a daisy chained cable configuration reduces cable clutter. Integrated controllers and drivers mean Zaber products are ready to go out-of-the-box, with no need to source and configure additional components.
Chip positioning and Imaging
The Nucleus MVR inverted microscope integrates XY sample positioning, focus and illumination control into a single system. The integrated illuminators support both transmitted brightfield and epifluorescence imaging. Digital IO enables precise triggering of cameras, yielding tightly coordinated control of the positioning, transmitted illumination and imaging of the microfluidic chip. This highly coordinated operation makes it easy for advanced imaging techniques like stroboscopic and moving shot imaging to be easily employed, eliminating the need for expensive high-speed cameras.
More detailed information on how to automate microfluidic chip positioning is available in our [Automating Chip Positioning article].
More detailed information on how to automate microfluidic imaging is available in our Automating Imaging for Microfluidics article.
Fluid delivery
To supply the chip with a steady flow of fluids, Zaber opted for a syringe pump design. The pump was constructed using a Zaber X-LSM linear stage with a 3D printed mounting bracket for the syringe. The X-LSM100A linear stage has a minimum speed and speed resolution of 29 nm/s which enables it to deliver flow rates as low as 100 pL/s with the appropriate syringe. At its maximum speed with a large volume syringe, this pump can deliver up to 10 mL/s. Syringes are inexpensive and can easily be exchanged, eliminating contamination concerns as different reagents are used. The flexibility and low cost of this design is ideal for prototyping systems, though the limited volume of syringes means this approach would not be recommended for production devices.
More detailed information on how to automate, build and operate a linear stage-driven syringe pump is available in the Automating Fluid Delivery for Microfluidics article.
Camera
A Teledyne FLIR BFLY-U3-23S6M-C camera was used for image acquisition. The 1/1.2” image sensor format matches the Nucleus MVR microscope's field of view well. The camera’s global shutter readout minimizes distortion from imaging moving droplets. I/O triggering provides an easy path to setting up tightly coordinated image acquisition, chip positioning and illuminator control. This coordinated operation enables the camera to function as a cost-effective alternative to more expensive high speed or scientific CMOS cameras.
System configuration
To operate the droplet generator microfluidic chip, the continuous phase and dispersed phase were supplied by two X-LSM syringe pumps. The fluids used were 2% flowSurf in HFE 7500 at 0.4 μl/s and dyed water at 35nL/s, respectively. The syringe pumps were daisy chained to each other through the MVR microscope, enabling the pumps,the integrated illumination, and motion control systems of the MVR to be controlled over a single USB connection.
The Python Zaber Motion Library was used to automate the control of the Zaber devices providing fluid delivery, sample positioning and illumination. OpenCV was used to perform the image processing required for the guidance of the system’s automated chip positioning.
Results
The performance of the system was evaluated based on images of droplets (Fig. 2) produced by the droplet generator on the sample chip.
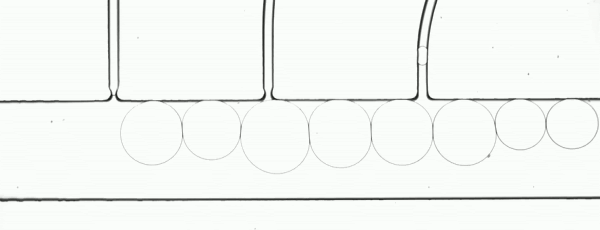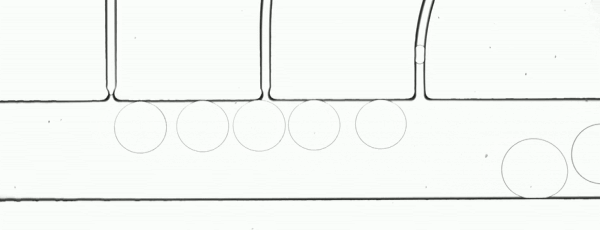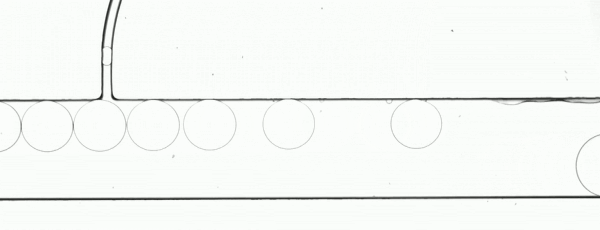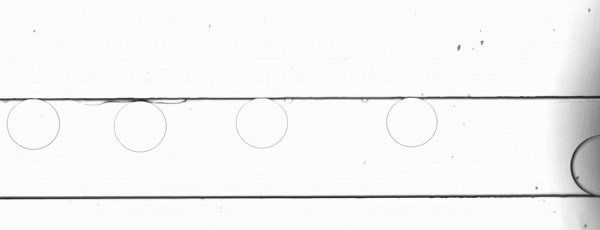
Figure 2. Consistent droplet sizes demonstrate a steady flow rate of the linear stage-driven syringe pumps, while the sharp images captured using a moving shot imaging technique demonstrate the effectiveness of the illumination, chip positioning and imaging acquisition control of the MVR microscope.
The example automated microfluidic system performed extremely well and produced droplets of a consistent size at a consistent rate. The images which enabled the measurement of the system’s performance were detailed and unambiguous. The results of this demonstration clearly show the suitability of the Zaber MVR microscope and X-LSM-driven syringe pumps for the development of automated microfluidic systems for research and diagnostic development purposes.
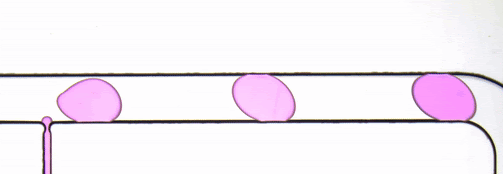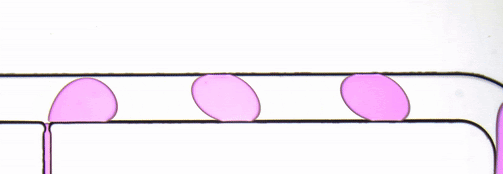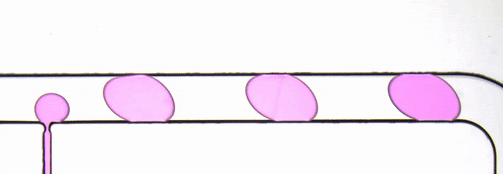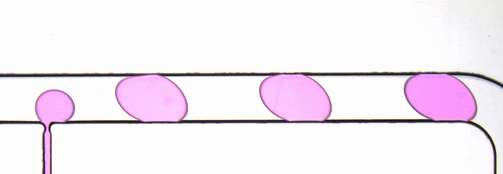
Figure 3. Droplet generator imaged with transmitted illumination using a Nucleus MVR microscope.
